Pressure and partial pressure are also important parameters in the distillation field.
Partial pressure : The pressure that would be exerted by one of the gases in the mixture if it alone occupied the volume of the mixture at the same temperature.
There are several useful laws that are related to the partial pressure,
For example:
1) Raoult's Law : It states that the partial pressure of the component i in an ideal mixture is equal to the product of mole fraction of that component i in the mixture and its vapour pressure of the pure component i.
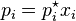
It is also important to know what does an ideal mixture exactly mean. There are two different types of mixture. One is ideal and another one is real mixture. There is no such thing as ideal mixture in reality. However some mixtures get quite close to being ideal. These are the mixtures of two or several molecules that are fairly similar to one another.
Examples of ideal mixture:
1) Hexane and cyclohexane
2) Hexane and Heptane
3) Benzene and Toluene
2) Henry's Law : It states that the amount of gas dissolved in a liquid at one particular temperature is directly proportional to the partial pressure of the gas which is in equilibrium with that liquid.

It is worth mentioning that Henry's law is valid for an ideal dilute solution. This means it is applicable to a mixture where the solvent is in excess and the mole fraction of the component is approaching zero. Henry's constant in this gas law depends on type of solute, temperature and pressure.
Partial pressure : The pressure that would be exerted by one of the gases in the mixture if it alone occupied the volume of the mixture at the same temperature.
There are several useful laws that are related to the partial pressure,
For example:
1) Raoult's Law : It states that the partial pressure of the component i in an ideal mixture is equal to the product of mole fraction of that component i in the mixture and its vapour pressure of the pure component i.
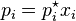
It is also important to know what does an ideal mixture exactly mean. There are two different types of mixture. One is ideal and another one is real mixture. There is no such thing as ideal mixture in reality. However some mixtures get quite close to being ideal. These are the mixtures of two or several molecules that are fairly similar to one another.
Examples of ideal mixture:
1) Hexane and cyclohexane
2) Hexane and Heptane
3) Benzene and Toluene
2) Henry's Law : It states that the amount of gas dissolved in a liquid at one particular temperature is directly proportional to the partial pressure of the gas which is in equilibrium with that liquid.

It is worth mentioning that Henry's law is valid for an ideal dilute solution. This means it is applicable to a mixture where the solvent is in excess and the mole fraction of the component is approaching zero. Henry's constant in this gas law depends on type of solute, temperature and pressure.

No comments:
Post a Comment